Genotox
Human cell based High-throughput Genotoxicity Screening Assay
(Patent information: WO 2012137186 A1)
In vitro and in vivo methods are available to detect compounds which may induce genetic damage either directly, or indirectly. Registration of pharmaceuticals requires the testing of these compounds using a standard battery of tests for detecting genotoxicity. Currently, the recommended tests comprises: 1) Ames test (reversion of mutations in bacteria), 2) In vitro cytogenetic evaluation of chromosomal damage with mammalian CHO cells, or mouse lymphoma tk assay, and 3) In vivo test for chromosomal damage using rodent hematopoetic cells. These tests are time consuming, and expensive; therefore these are often done at late stages of preclinical drug development. Additionally, each of these tests has drawbacks that may result in false results. Since the replication and DNA damage repair mechanisms are very different in bacteria and mammalian cells, the Ames test may at times be inappropriate. The Ames test has shown to be inadequate in examining some antibiotics, and compounds known to interfere with mammalian cell replication. There are even compounds where standard in vivo tests do not provide enough data regarding genotoxicity; examples include compounds that not systemically absorbed and not available to the target tissues – some radioimaging agents, aluminum based antacids, topically applied pharmaceuticals. The reasons stated above, and the increasing number of diseases associated with genotoxicity worldwide prompted the initiation of a project for a rapid, cell based reporter assay, anthem genotoxicity screening assay that could be used even in early phases of discovery.
Genotoxicity screening assay article
Human Cell Based High-throughput Genotoxicity Screen News
Genotoxicity screening assay article
Human Cell Based High-throughput Genotoxicity Screen News
Genotox screen
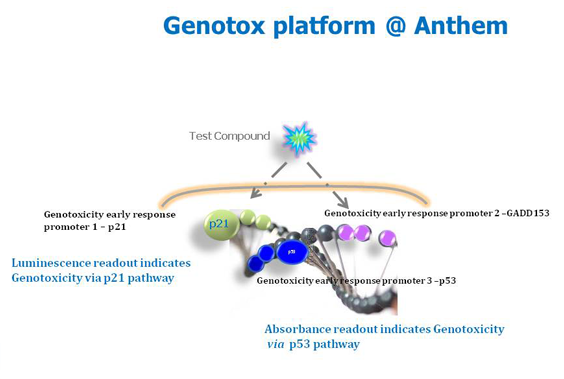
Various types of DNA damage that includes oxidation, alkylation, hydrolysis of base and adduct formation occurs via endogenous cellular processes. UV-A/B, ionization radiation and a large number of compounds present in the environment (pollutants), natural products, and chemically synthesized for use as pharmaceuticals, food additives, and in agriculture are examples of exogenous agents that causes damage to DNA. Cells exposed to the agents respond to these DNA damaging agents using sensor proteins such as ATM, ATR, the Rad17-RFC complex, and the 9-1-1 complex. These proteins initiate p53 dependent and independent pathways that results in the activation of many genes involved in DNA repair and cell cycle progression. There is ample evidence from literature that DNA damage is complex, and a single gene reporter will not be sufficient in sensing various types of damage. Anthem has therefore engineered a three reporter system for sensing genotoxic stress that will sense both p53 dependent and independent effects.
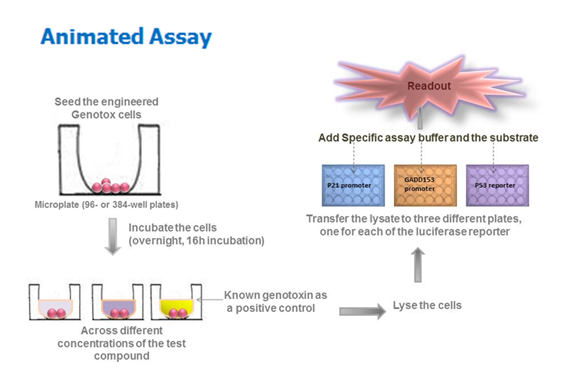
Features of Anthem genotoxicity screening assay
- It is an engineered human cell line based screen that is compatible for high-throughput analysis.
- The same cell line reports four parameters – cytotoxicity, and three genotoxicity read-outs.
- The amount of compound required is minimal ~ 10 mg/compound
- The assay can be completed and analyzed in 2 days, versus weeks for other conventional assays
- Extensive validations have been conducted in-house, at external sites and in collaboration with labs in Europe (the data has been published *).
- The sensitivity and specificity values far exceed any of the standard assays currently in use.
- It is the best in class assay if concordance with regards to in vivo data.
Grants & Journals







